Mastering the Basics: A Comprehensive Guide to the Fundamentals of Thermodynamics
Thermodynamics
Thermodynamics is a fundamental branch of engineering science that deals with the study of energy, heat, and work, and their interactions within systems. In mechanical engineering, thermodynamics provides the foundation for understanding how energy is transformed and utilized in various machines and processes. Indian mechanical engineers often apply thermodynamic principles in the design, analysis, and optimization of engines, refrigeration systems, power plants, and other energy-conversion systems.
In India, thermodynamics plays a crucial role in industries like power generation, automotive manufacturing, aerospace, and renewable energy sectors. Engineers leverage the principles of thermodynamics to develop sustainable and efficient solutions, crucial for the nation's growing energy demands and industrial advancements.
Fundamental Terms in Thermodynamics
Thermodynamic System
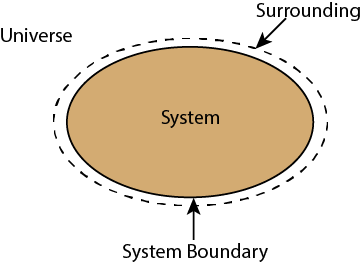
The thermodynamic system is a bounded region where thermodynamic processes get executed under observance. Let us consider an example of the cricket ground to understand the concept of a thermodynamic system. During a cricket match, players perform on the field, and the audience watches the game. If we consider the fence of a cricket field as an enclosed region and the audience observes the process, then the cricket field would be the system.
Open System
The system which allows transfer of both Mass and Energy across the boundary is known as Open System.
Closed System
The system which allows transfer of energy across the boundary, but doing not allows transfer of mass across the boundary.
Isolated System
An isolated system is one which does not allow transfer of both energy and mass across the boundary. There is no such any perfect isolated system exists other than universe.
Let us have a look at summary of types of system in the form of table,
| System | Transfer of Energy | Transfer of Mass | Example |
|---|---|---|---|
| Open System | Allow | Allow | Pouring Tea into the saucer from cup. |
| Closed System | Allow | Do not Allow | Tea in Open Cup |
| Isolated System | Do Not Allow | Do Not Allow | Thermos Flask, Ice Can, Universe |
Surrounding
The rest portion of the universe excluding the system is known as surrounding.
Boundary
The imaginary line between system and surrounding is known as boundary. Usually, boundary separates the system from the surrounding.
Laws of Thermodynamics
There are four fundamental laws of thermodynamics exists, let us have a look on each separately,
Zeroth Law of Thermodynamics
According to the Zeroth Law of Thermodynamics, “If two bodies are in thermal equilibrium with the third body separately, then these two bodies must be in thermal equilibrium with each other.”
Let us take an example of three bodies such as Body A, Body B, and Body C. If Body A is in thermal equilibrium with Body C, and Body B is also in thermal equilibrium with Body C, then A and B must be in thermal equilibrium with one another.
In addition, thermal equilibrium refers to the property of the system that allows heat transfer, but even after the heat transfer, no significant change in the state occurs. This principle enables the application of a thermometer (body C) to measure the temperature of systems A and B and confirm that they have the same temperature without directly comparing them.
First Law of Thermodynamics
The First Law of Thermodynamics can be expressed through various relevant statements, each emphasizing different aspects of energy conservation and transformation. Here are the key statements related to the First Law:
Energy Conservation Statement
Energy cannot be created or destroyed, only transformed from one form to another. This is the fundamental principle behind the First Law and applies universally in all thermodynamic processes.
Change in Internal Energy
The change in the internal energy of a system is equal to the heat added to the system minus the work done by the system.
Mathematically,
∆U = Q - W
Where,
ΔU – Change in internal energy
Q – Heat - The energy transferred due to a temperature difference between the system and its surroundings.
W – Work done - The energy transferred when the system does mechanical work (such as moving a piston, turning a turbine, etc.).
For Cyclic Process
In a cyclic process (where the system returns to its initial state), the net heat supplied to the system is equal to the net work done by the system.
∮dQ= ∮dW
In other words, the cyclic integral of change in Heat is equal to the cyclic integral of work done. Since the system returns to its initial state, there is no change in internal energy over a complete cycle, making the heat input equal to the work output.
These various forms and statements of the First Law of Thermodynamics are crucial for analyzing a wide range of mechanical systems, from engines and turbines to refrigeration and heating systems, ensuring that energy balances are accurately maintained in engineering applications.
Applications of First Law of Thermodynamics
Internal combustion engine is one of the best examples of application of first law of thermodynamics.
• Fuel combustion adds heat energy to the engine (Q).
• Part of this energy is converted into mechanical work (W) to move the pistons.
• The remaining energy increases the internal energy of the engine components or is lost as heat to the environment.
Second Law of Thermodynamics
The Second Law of Thermodynamics introduces the concept of irreversibility in natural processes and establishes the direction in which processes occur. It can be stated in several ways, but all highlight that energy transformations are not 100% efficient.
Entropy Statement
In an isolated system, the total entropy (a measure of disorder or randomness) can never decrease; it either remains constant for a reversible process or increases for an irreversible process.
This indicates the natural tendency of systems to move towards greater disorder or randomness over time. In any real-world process, entropy always increases, meaning that energy transformations are irreversible and some energy becomes unavailable to do work. Note that there is no any perfect isolated system available other than the universe.
Kelvin-Planck Statement
It is impossible to construct a heat engine that operates in a cycle and converts all the heat absorbed from a heat source into work without rejecting some heat to a lower-temperature reservoir.
This means that no heat engine can be 100% efficient, as some energy must always be lost as waste heat. As shown in the graphical representation of the Kelvin-Plank Statement above, the heat transferred from the high-temperature reservoir (Source) gets converted to produce essential mechanical work by employing the Heat Engine, and then the rest of the heat gets transferred to the sink. It clearly shows that no 100% utilisation of heat energy from the source occurs throughout this process of obtaining the work output.
To produce work, a heat engine needs a temperature difference between a hot source and a cold sink. Heat must flow from the hot source to the cold sink, and only part of the heat flowing through the engine can be transformed into work. This is why every engine requires a cooling system (e.g., the radiator in a car engine) to dispose of waste heat. The efficiency of any heat engine is always less than 100% because, the temperature of the cold reservoir, is always greater than zero. Hence, some heat must always be rejected.
Clausius Statement
It is impossible to construct a device that operates in a cycle and transfers heat from a colder body to a hotter body without any external work being done.
This statement explains why heat naturally flows from hot objects to cold ones and why refrigeration systems require external work (like electricity) to move heat from cold to hot.
The Second Law of Thermodynamics mandates that energy transformations are inherently inefficient due to the production of entropy and irreversibilities. Thus, it's impossible to convert all heat into work in any thermodynamic process, because some heat must always be rejected to a colder reservoir. This is why 100% energy conversion efficiency is theoretically and practically unattainable.
Third Law of Thermodynamics
The Third Law of Thermodynamics deals with the behavior of systems as they approach absolute zero temperature. It can be stated as:
"As the temperature of a system approaches absolute zero (0 Kelvin), the entropy of a perfect crystalline substance approaches zero."
Absolute zero is the theoretical lowest possible temperature, defined as 0 Kelvin (−273.15°C or −459.67°F). At this temperature, a system has minimal energy, and the thermal motion of particles finishes. A perfect crystal is an idealized solid material where the atoms are arranged in a perfectly ordered structure, with no defects or randomness. In such a crystal at absolute zero, there is only one possible microscopic arrangement of atoms, meaning that the system has only one microstate and therefore zero entropy.
Entropy (S) is a measure of disorder or randomness in a system. According to the Third Law, as temperature decreases, the randomness or disorder decreases because the energy available for particles to move becomes smaller. At absolute zero, a perfect crystalline material would have no disorder because the particles are in their most ordered, lowest-energy state. Therefore, the entropy of such a system becomes zero:
S→0 as T→0
However, in real systems, there are usually some imperfections, so absolute zero entropy is approached but never fully achieved. The Third Law of Thermodynamics sets an important limit in thermodynamics by defining the behavior of entropy as a system approaches absolute zero. While it is impossible to reach absolute zero, the law provides a framework for understanding the limits of cooling processes and entropy at low temperatures. It plays a critical role in cryogenics, low-temperature physics, and material behavior near absolute zero.






Comments